Predesulphurization of Naphtha process description:
Hydrogenation and stripping are the primary processes to remove bulk sulphur present in naphtha. Sulphur is a harmful component in raw naphtha when used as primary stock in ammonia production. Sulphur is treated as an impurity which has a huge impact on the production cost of ammonia. It is a poisoning element for steam naphtha reforming catalyst it should be removed from raw naphtha.
The process involved in the removal of sulphur impurity is called Pre-desulphurization since another final desulphurization system is been established in present process ammonia manufacturing plants along with PDS because of the availability of raw naphtha containing sulphur of about 1500 ppm which is not desirable to present technology catalyst. This fraction is reduced to 10 to 5 ppm in Pre-desulphurization, and final desulphurization is used to absorb the remaining traces of sulphur.
Unit operation equipment used in this process are:
- Deaerator
- Recycle compressor
- Feed effluent heat exchangers
- Fired heater
- Hydrogenator (fixed packed bed reactor)
- Effluent separator
- Naphtha stripper
The raw naphtha is passed through a deaerator to remove oxygen content dissolved in the liquid naphtha using natural gas or naphtha vapours, this operation will prevent the risk of gum formation in heat exchange equipment. The stripped off-gas from the deaerator is used as fuel and the liquid naphtha from the deaerator is pumped through a series of effluent heat exchangers to vaporize and then to the superheated fired heater. Sulphur present in the form of mercaptan, thioether, and disulphide is made to react with hydrogen and form hydrogen sulphide at Hydrogenator. The reaction is exothermic; the heat obtained is used to preheat the inlet feed to the fired heater before the reactor to attain the reaction temperature. Hydrogen gas is added at the compressor section to the liquid stream of deaerated naphtha. The mixture at 40 kg/sq.cm pressure is passed to the effluent heater and vaporized to a temperature of 300degC. This temperature is raised to 380 deg C by the fired heater and passed to the top side of the fixed packed bed catalytic Hydrogenation reactor. Most of the sulphur is reacted with hydrogen as per equation.
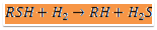
Where R, is a radical of hydrocarbon.
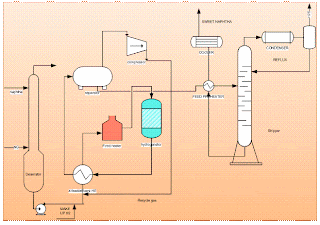
Process flowsheet of predesulphurization plant
The reaction mixture is cooled to separate the naphtha and hydrogenated gas. Liquid naphtha which is cooled is fed to a stripper to strip out dissolved water and hydrogen sulphide. The stripper feed preheater is used to preheat the feed naphtha by the naphtha stream obtained from the bottom of the stripper which is at a temperature of about 200-250 deg C. Stripper is a distillation column having around 20 trays and operates at 10kg/sq cm. Temperatures are maintained around 200 to 130 deg C at the bottom and 130 to 90 deg C at the top depending on feedstock. Off gases are used as fuel and the naphtha obtained at the bottom is called sweet naphtha. Sweet naphtha should contain less than 15 ppm of total sulphur.
Related Topic click here
- SULPHATES Determination Test | Sulphites Determination | Determination Of Sulphate And Sulphide In Water
- Petrochemical Products From Propylene | Types Of Pressure Gauges | Types Of Reactors Used For Chemical Reactions And Chemical Process
- Printing Machine And Devices
- Phosphoric Acid Per Day Flow Sheet Of Dihydrate Process
- Types Of Phenol Manufacturing Process
- Chlorobenzene And Caustic Process For Phenol Production | Phenol Production By Benzene Sulfonation Process
- Bin Blender Applications
- Operating Procedure For Liquid-Liquid Extraction In Packed Column



