Naphtha reformer for Hydrogen production
To reform natural gas or naphtha which are rich sources of hydrocarbon are reformed to produce Hydrogen using reforming equipment that consists of side-fired radiant burners that externally heat feedstock. There will be about six rows of burners across four sides and 15 burners in each row. Feed passes through the catalyst bed, and CH4 (Methane) or Naphtha vapors which are converted to methane react with steam to give Hydrogen and carbon dioxide.
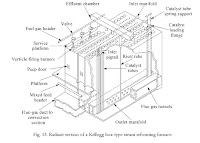

Natural Gas Plant Design and Process Description
Natural Gas is a combustible gas that is available in the earth’s crust, mainly under the rocks where there is a petroleum deposit. Natural Gas will be along with crude petroleum and in some cases, it exists individually. Natural Gas is used as an energy source next to liquid petroleum and solid coal, it is an important raw material for the production of petrochemicals. The general composition of Natural Gas contains 70 to 98% of Methane, 16% of alkanes from Ethane to Hexane, and 15% of Nitrogen, Carbon dioxide, and Hydrogen Sulphide.
Natural Gas Processing has direct and indirect paths to get various derived chemicals and energy.
- Direct method Natural Gas is combusted or partially combusted without the catalyst to form Formaldehyde, C2H4, C2H6, CO, and H2.
- By the Indirect method, Natural Gas is processed to produce a SYNTHESIS GAS. where all the hydrocarbons are converted to Hydrogen and Carbon-monoxide mixture, this becomes a starting material for the production of various petrochemicals like Ammonia, Methanol, Acetic acid, Phosgene, Oxo alcohols, metal carbonyls, hydrocarbons. Steam reforming is the technique used for the production of synthesis gas from natural gas.
Steam reforming of Natural Gas:
Natural gas and Steam are reacted on a catalyst at operating conditions leading to the production of synthesis gas, the reaction on the catalyst(Ni6 Al2(OH)16. CO3.4H2O) would be like
CH4 + H2O <=> CO + H2
Related Essay
- PH Measuring Instruments, Working Principle, Theory, And Determinating Methods
- MULTI-COMPONENT DISTILLATION COLUMN DIAGRAM | Nanobots And Nanotechnology
- Spark Plug Maintenance And Function In Performance Of Automobiles | Motorcycle Maintenance Workshops And Services
- Production Process Options For High-Impact Polystyrene
- Types And Modes Of Heat Effects | Individual Heat Transfer Coefficient And Thermal Diffusivity Calculator
- Hydrogen Production And Hydrogen Storage Techniques
- Flow Through Packed And Fluidized Beds | Fluid Mechanics
- Fluid Catalytic Cracking Unit Flow Sheet And Process Equipment



