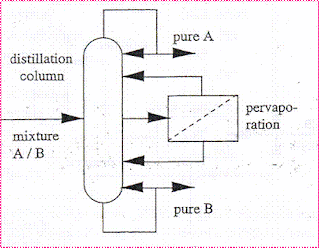
A setup for the distillation column and evaporator
By combining distillation and pervaporation a Hybrid system become more economical and energy efficient. we can eliminate the additional trainer and equipment for azeotropic distillation systems.
Distillation column trays can be decreased. The purity requirement is achieved. Byproduct removal and reactant recycling in Reactive distillation can be handled. It is the arrangement of equipment to obtain the optimized model for the desired separation.
Operating parameters can be optimized like :
No trays, feed tray location, reflux ratio, permeate and retentate recycle ratio, membrane feed location, and a number of membrane modules are required. These are optimized for each superstructure’s configurations.
A superstructure of the hybrid process for azeotropic separation and reactive distillation will be like this.
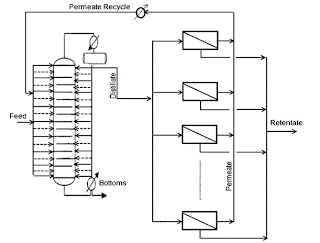
Distillation and Distillation Column
How does mass transfer occur in a distillation column and how the concept of distillation has become so important in the purification and separation process are interesting topics for chemical engineers. Designing a distillation column should be known to all the fresh engineers after the completion of their course study. Ok, how do we define a distillation operation? A simple definition is “Distribution of substances or components between a gas or vapour and liquid phase”. This evolves the objective of adjusting the phases.
One thumb rule to be memorized is that distillation means the heat content (heat relation of substances) i.e. energy property related to the mass percentage. The advantage of the distillation operation is that it is the ultimate purification process for chemical products. That is why it dominates all mass transfer operations.
The latest research around the world is focused on the packing structure, material of construction and plates for stage contacts. The application of heat plays an important role in the distillation process which is why thermodynamic properties data are required (which include the heat capacity data at particular temperatures) and can be obtained for the research papers and standard data packages and materials.
Before learning distillation operation one should gain knowledge of the concept of EQUILIBRIA. From physical chemistry, this concept is explained in detail which includes the equilibria which exist between liquid and vapour phases, and the properties of phases of each compound. It explains the graphs that show the relationship between temperature, vapour pressure, and concentration of the compounds.
This information is sufficient for the design equation of equipment used for the distillation of required components. By applying simple material and energy balance over the process equipment, they optimize the flow rates, heat to supplies, condenser capacity, and the number of stages required. McCabe-Thiele method and the Ponchon-Savarit method are frequently used for the estimation of calculating the number of theoretical plates of a distillation column based on both material and enthalpy balances.
If Rm is the minimum reflux ratio the optimum reflux ratio is 1.25 to 1.5 Rm. Rayleigh equation applies to differential distillation. If the feed to a distillation column is introduced onto a plate one plate below the feed plate, the total number of theoretical plates required for the desired separation will increase.
There is a relationship between the reflux ratio used in a distillation column and the column diameter as the reflux ratio increases, the column diameter increases. Azeotropic distillation is a special case of multicomponent distillation for azeotropic separation of the acetic acid-water mixture the entrainer that is employed is n-butyl acetate and for azeotropic separation of the ethanol-water mixture, the trainer that is used isobenzene.
Types of Distillation Operations
- Flash Distillation
- Steam Distillation
- Simple or differential Distillation
- Continuous Distillation
- Azeotropic Distillation
- Extraction Distillation
- Vacuum Distillation
- Distillation with Pervaporation
Distillation at reduced pressure is practised for the separation of heat-sensitive materials only and components having low volatilities at normal pressures. The minimum number of plates required for the desired separation by distillation does not depend on the thermal condition of the feed.
To formulate calculations describing distillation operation, knowledge of vapour–liquid equilibria for the concerned system is a key requirement. Literature provides vapour–liquid equilibria data for various systems. In absence of this, the equilibrium data is computed from relative volatility ‘α’ as under
By definition
ALPHA(α) = Ya(1 – Xa)/Xa(1-Ya)
Where A refers to the more volatile component, from this a relation between Ya and Xa is obtained as
Ya = α × Xa/ [1+ (α -1 )Xa]
In the absence of data, the value of ALPHA can be computed from vapour pressure data of the components as,
α ab = Pa/ Pb
a and b are two components in the mixture.
FLASH DISTILLATION:
In this case, a definite fraction of liquid is vaporised in such a way that the evolved vapour and residual liquid are in equilibrium with each other. The process is described by
Ya= – [1-f/f]Xa + X Fa/ f
Where f= molar fraction of feed vaporised.
Differential (simple) distillation: In this case, the vapour is removed as soon as it is formed without appreciable condensation. The course of a differential distillation process is given by Rayleigh equation.
Related topic
- Electrical Transformers | Switchgear Protective Device | Power Consumption Of Appliances And Electrical Apparatus
- Simple And Differential Distillation Experiment Procedure
- Distillation Column Diagram | EFFECT IN TOWERS AND COLUMNS
- Hybrid Distillation And Pervaporation System | Distillation And Distillation Column
- How To Design And Construct A Distillation Column Along With Mechanical Parameters? What Are The Stepwise Procedures For Designing The Distillation Column?
- An Introduction To Diffusion Concept In Mass Transfer
- Flow Through Packed And Fluidized Beds | Fluid Mechanics | Flow Through Packed And Fluidized Beds
- Fluid Catalytic Cracking Unit Flow Sheet And Process Equipment | Fluid Catalytic Cracking (FCC)


